Usually three consecutive batches of medicines are necessary to affirm the good results of the process design and style and qualification stages.
Water for Injection— Water for Injection (see USP monograph) is utilized being an excipient in the creation of parenteral along with other preparations in which merchandise endotoxin articles have to be controlled, and in other pharmaceutical apps, for instance cleaning of particular equipment and parenteral product-Get in touch with parts. The least top quality of source or feed water for that era of Water for Injection is Ingesting Water as described through the U.S. EPA, EU, Japan, or the WHO. This supply water might be pre-addressed to render it well suited for subsequent distillation (or whatsoever other validated method is applied in accordance with the monograph). The finished water ought to fulfill the entire chemical needs for Purified Water together with an extra bacterial endotoxin specification. Due to the fact endotoxins are produced by the styles of microorganisms which can be prone to inhabit water, the tools and strategies employed by the system to purify, retailer, and distribute Water for Injection have to be created to minimize or protect against microbial contamination together with clear away incoming endotoxin from the starting up water.
Qualification things to do wherein spots need to be identified for placement of knowledge logger or sensors, then schematic layouts to depicts the place of sensors or area identification shall be specified in the Qualification/validation protocol for better clarity.
This stage makes certain that all tools has long been mounted the right way as meant, in accordance with criteria set from the maker, and with all vital documentation.
When carrying out their experiments from the laboratories, the experts will currently be contemplating the categories of kit that could be applied when the process would be to be scaled-up for business production of large quantities with the drugs.
Also, any components influencing how the choices about the method were being created also needs to be documented.
With present day tight time schedules, a Body fat is quite handy for the new installation of a plant. The edge is the fact premanufactured models are checked and examined as much as possible prior to They're despatched to web-site.
Feedback must be laid out in creating and compiled in a single doc clarifying who has commented on what. For rapid-track assignments, these approval routines are notably crucial and need to be founded at the beginning in the project. It is also advised that the volume of approving get-togethers is kept to your minimum amount. The person need to specify which regimen applies to modify requests while in the job and from when it really is relevant.
If all of our cake testing (together with screening the products and also the cake blend at a variety of points all over the approach) provides effects inside the vary our comprehensive recipe say they need to, we’ll move our PPQ.
The new qualification approach for cell water systems has long been mentioned with gurus and authorities from all all over the world. Working with it, the qualification process could be shortened to your period of less than three months from installation to launch from the water for pharmaceutical reasons. Cell water systems are feasible for different GMP apps and can help steer clear of purified water shortages in the pharmaceutical web site.
The 3rd segment, the provision or distribution loop, will be the distribution piping that provides the RODI Water for the details-of-use and returns the excess on the more info storage tank. As well as the common demands, the subsequent are necessary with the deionized water system.
Control of the organic and natural and inorganic impurities and microbiological quality of water is check here vital because proliferation of micro-organisms ubiquitous in water could arise in the purification, storage, and distribution of this substance.
We’ll also execute additional exams right here than we will at the time we’re routinely generating our cakes, mainly because we’re even now making an attempt to ensure our process and machines and manufacturing just what exactly they’re meant to.
Activated Carbon Granular activated carbon beds adsorb minimal molecular pounds organic substance and oxidizing additives, for instance chlorine and chloramine compounds, eliminating them from your water. They are really utilised to obtain sure excellent attributes and to shield in opposition to response with downstream stainless-steel surfaces, resins, and membranes. The Main functioning considerations about activated carbon beds contain the propensity to guidance bacteria advancement, the prospective for hydraulic channeling, the organic and natural adsorption potential, suitable water circulation premiums and speak to time, The shortcoming to get regenerated in situ, along with the shedding of micro organism, endotoxins, organic chemical substances, and fantastic carbon particles. Control actions may well contain checking water circulation costs and differential pressures, sanitizing with incredibly hot water or steam, backwashing, screening for adsorption capability, and frequent replacement of the carbon bed. In the event the activated carbon bed is meant for organic and natural reduction, it may additionally be proper to monitor influent and effluent TOC. It's important to notice that the usage of steam for carbon bed sanitization is commonly incompletely powerful on account of steam channeling as an alternative to even permeation with the mattress.
 Sam Woods Then & Now!
Sam Woods Then & Now!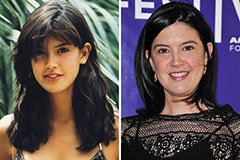 Phoebe Cates Then & Now!
Phoebe Cates Then & Now!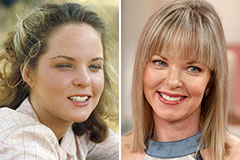 Melissa Sue Anderson Then & Now!
Melissa Sue Anderson Then & Now! Bo Derek Then & Now!
Bo Derek Then & Now! Ricky Schroder Then & Now!
Ricky Schroder Then & Now!